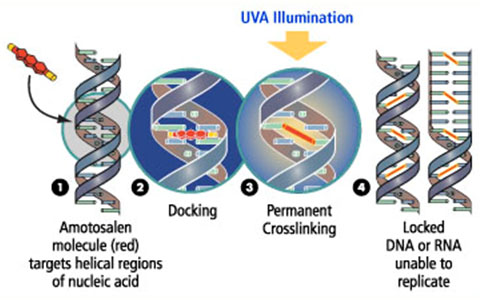
Pathogen Inactivation
Molecular and cellular effects of the pathogen inactivation process on platelets
In 2009, Swissmedic approved the Intercept process to inactivate pathogens in platelet concentrates, and made compulsory the use of a pathogen protection strategy in all Swiss blood banks before July 2011. The Intercept process for pathogen inactivation in platelet concentrates is now being deployed all over Switzerland, and the blood banks of Zurich, Basel and Lausanne adopted the process in early January 2011 in routine as pilot centres. Whereas there is ample evidence that the Intercept process efficiently protects the blood supply chain against a very large panel of yeasts, bacteria and viruses, its effect on platelet biomedical efficiency is still a matter of debate: in most clinical studies, platelets treated with the Intercept process appeared to be somehow less efficient in restoring or maintaining the haemostatic capacity of (mostly onco-haematological) patients. The gist of the present project is to explore at a molecular and cellular level the deleterious effects of the Intercept and other pathogen reduction technologies (PRT) processes on platelets.
On the long track, we aim at better understanding the pathogen inactivation process itself and its direct deleterious effects on platelets, help clinicians formulate recommendations for the use of treated platelets, and provide objective elements to orientate the choice of PRT in the future (either as other technologies become available for platelet concentrates or for other labile blood products).
Our research is mainly divided in two topics:
•Impact at the molecular level (metabolites, peptides, proteins)
•Impact at the functional level (adhesion and aggregation of platelets)
Oxidative damages on peptides
We evaluated the behavior of the two main PRTs, namely Intercept and Mirasol (using a combination of UVA-B / riboflavin instead of UVA / amotosalen) at the peptide level (Prudent et al., JASMS, 2014). Several studies have demonstrated a decrease in platelets functionality and efficiency after the transfusion due to these PRTs. Because these photochemical treatments imply oxidation processes and the generation of reactive oxygen species (ROS), the strategy was to first find out the potential oxidations of amino acids due to PRTs, to classify and quantify them, on model peptides by LC-MSMS analysis. Hence, we identified different types of oxidations for all tested peptides containing the following amino acids: Cys, His, Trp or Tyr. Moreover, this work demonstrates that the Intercept and Mirasol may impact differently the oxidation of peptides/proteins.
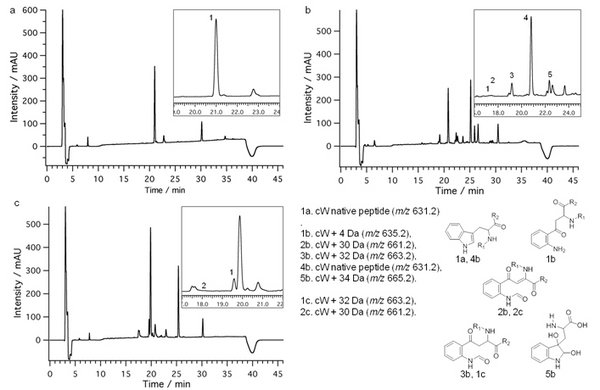
Below is an example of AAAWAAA (cW) model peptide.
UV chromatograms at 214 nm of cW +UV only (a), amotosalen/UVA treated (b) and riboflavin/UVB treated (c). See inset and legend for peak details. Other main peaks correspond to degradation products of amotosalen or riboflavin. Modified tryptophan structures due to PRTs are proposed on the bottom right.
In a near future, these results will be included in proteomic database searches and will allow to specifically target protein modifications with a better matching.
Proteomic analysis of Intercept-treated platelets
A gel-based proteomic strategy was applied to study the effect of Intercept treatment on platelet concentrates from pooled buffy-coat (Prudent et al., J. Proteomics, 2012). Samples from five PCs (10 donors per PC) were withdrawn at day 1 (before treatment) and at days 2 and 8 from the treated and untreated PC. Total protein extracts were separated on 2DE (pI 3-10) and spots of interest were analyzed by LC-MS/MS for protein identification.
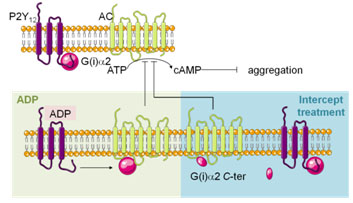
The detailed analysis of the 2DE maps reveals the low impact of the Intercept treatment on the proteome. Only three proteins were affected by the Intercept treatment: DJ-1, glutaredoxin 5 and guanine nucleotide-binding protein G(i) subunit a2 (G(i)a2). As for the impact of storage, three other proteins were over-expressed with time and independently of Intercept treatment: chloride intracellular channel protein 4 (CLIC4), actin and cofilin-1.
Functional lesions to treated platelets
In this part, we aim at characterizing the functional lesions of Intercept-treated platelets at different levels.
The adhesive properties of platelets on classical ligands are currently under investigation. The objective is to test adhesive behaviour of platelets on immobilised von Willebrand factor (platelet receptor GPIb-V-IX), immobilised fibrinogen (platelet receptor aIIbßIII), and immobilized collagen (platelet receptors a2ß1 and GPVI) in a static manner with microscopic investigation of adhered platelets, or in combination with colorimetric detection.
We are also investigating the aggregation behaviour of platelets in response to several agonists such as (Prudent et al., Transfusion, 2012):
•Thrombin (probe of PAR receptors outside-in signalling)
•ADP (probe of P2Y1 and P2Y12 receptors outside-in signalling)
•Thromboxan (probe of Thromboxan receptor outside-in signalling)
•Collagen
Experiments are performed on a APACT 4000 with 4 agonists: arachidonic acid (AA), thrombin receptor activating peptide (TRAP), collagen and ADP.
Concentrations of agonists are screened and adapted in order to highlight differences between treatment and control (above a certain limit eq. max aggregations were reached).
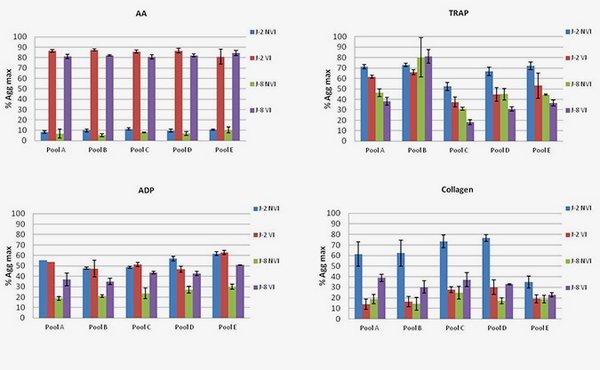
Platelet aggregation following the addition of different agonists. Differences between Intercept-treated platelet (VI) and untreated (NVI).
AA shows an increase in max aggregation after treatment and on the other hand TRAP assays highlight a decrease both at Days 2 and 8. No evidence was reported at the protein level but taking into account both effects, modifications could occur independently within the AA and TRAP pathways.
ADP shows an increase in aggregation only at day 8. ADP pathway seems affected, which could be correlated to the modification of G(i)a2 observed in proteomics. Such a conclusion remains hypothetical and will have to be investigated.
RBC Storage
Stored red blood cell aging
In standard SAGM additive solution, ECs are stored for 42 days at 4°C. This limited storage duration has a direct implication for blood banks: not all (serocompatible) ECs can be available at any time, and near-shortage situations can occasionally occur, which means that in Lausanne, approximately 10% of delivered ECs are bought from other blood banks. More importantly, there is accumulating evidence that receiving “old” blood (stored for more that 14 days for example) is not as advantageous as receiving fresher blood. For example, Koch et al clearly demonstrated that the life expectancy at 5 years is better for patients who received fresh blood (less than 14 days) compare to older blood in the course of cardiac surgery (Koch et al., NEJM, 2008). Even though the study is controversial and biais were present, the question is totally opened. Moreover, it is current practice that some clinical practitioners prefer to transfuse fresh blood (such as in neonatology for example) and put blood banks under pressure to prioritize delivery of fresher ECs. At the same time, relatively little is known about the molecular bases of the so-called “blood storage lesion”, and even less about their possible biomedical consequences; blood bankers are thus in an uncomfortable situation, where the general demand of ECs increases every year, the number of ECs produced does not increase as fast as the demand (at least in Vaud), and there is an increasing clinical pressure to prioritize the delivery of ECs depending on their storage duration. When facing this increasing pressure, blood bankers have very few arguments to evaluate the safety and efficacy of older products compared to fresher ones.
Our group aim at studying one of the major known pathways of RBC senescence and clearance: the Band 3 clustering model. In particular, we propose to document features of RBC alteration in storage conditions compared to known senescence processes in vivo. Moreover, this study will be performed with ECs stored in the main additive solutions used in routine in Switzerland: SAGM (sodium-adenine-glucose-mannitol) and other additive solutions, to establish if the use of different additive solutions induces significant molecular differences in RBCs. In addition, storage under anaerobic conditions will be evaluated.
Here is listed the different targets we investigate:
•Microvesiculations and effect on transfusion
•Protein content and protein oxidation
•Evolution of metabolites
Red blood cell storage lesion and microvesiculation
Microvesicles are small corpuscles of less than 1 μm that are released by RBCs through blebbing mechanisms involving cytoskeleton remodeling and membrane asymmetry disruption, with the exposure of phosphatidylserine at the outlet of the plasma membrane (and thus at the surface of microvesicles). We developed flow cytometric procedures to count microvesicles based on labeling with Annexin V (phosphatidylserine exposing microvesicles – PS+) or classical CD47 or CD232a. Strikingly, whereas no dramatic change in phosphatidylserine exposure or intracellular calcium concentration could be measured on red blood cells during storage (data not shown), the number of microvesicles present in blood bags continuously increase throughout storage (Rubin et al., Vox Sanguinis, 2008).
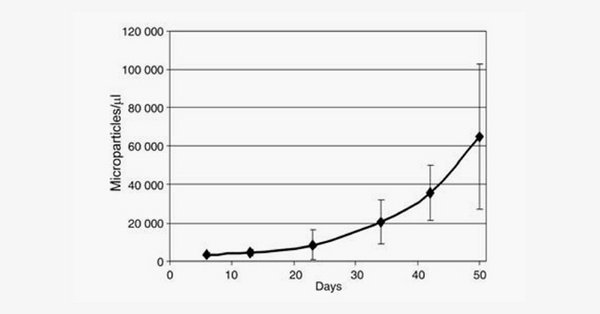
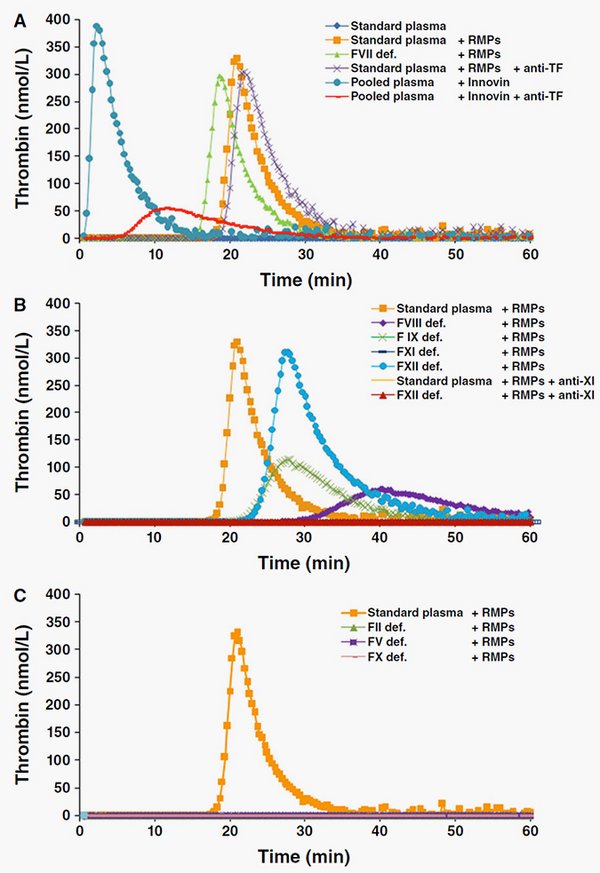
Because microparticles are bioactive, it could be suggested that MPs are mediators of posttransfusion complications or, on the contrary, constitute a potential hemostatic agent. Using calibrated automated thrombography, we investigated whether MPs affect thrombin generation (TG) in plasma (Rubin et al., Transfusion, 2012).
Based on these data, we have shown that MPs have FXI-dependent procoagulant properties and are able to initiate and propagate TG. The anionic surface of MPs might be the site of FXI-mediated TG amplification and intrinsic tenase and prothrombinase complex assembly.
Protein carbonylation during storage of erythrocyte concentrates
Red blood cells undergo storage lesions during transfusion-purposed storage.
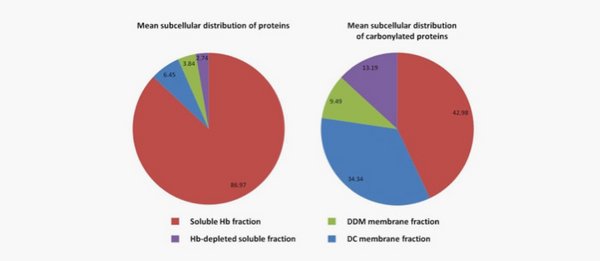
Oxidative stress plays an important role, altering and degrading the protein content, and is thought to involve RBC clearance through membrane modifications. Protein carbonylation is a marker of oxidative stress and aging, and has been shown to increase during RBC storage.
We have developed a subcellular fractionation to assess this modification in more details, and we propose a pathway leading to RBC microparticulation (Delobel et al., J Proteomics, 2012).
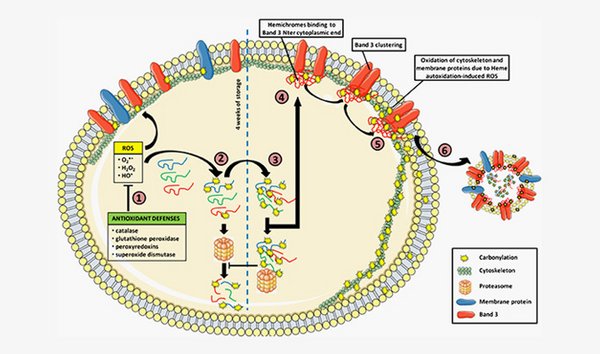
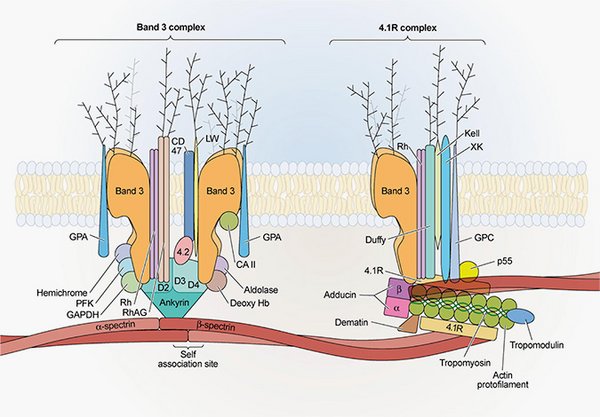
Evolution of band 3 complexes during storage of erythrocyte concentrates
The gist here is to isolate and identify partners within band 3 complexes from RBC. The band 3 protein is, in addition of being an anion exchanger, the site of macromolecular assembly for cytoskeleton anchorage, glycolytic enzymes, and regulatory proteins. These protein complexes, and thus the RBC functions, are potentially altered during storage. Such approach will provide information on the storage and the impact on transfusion as well as on the fundamental mechanism involved in RBC aging.
Red blood cell bioreactors: study of metabolism and proteomic analyses under different storage conditions
A mini-bioreactor has been developed in our lab in order to study the metabolism and the proteome of RBC in function of different storage conditions. The goal is to get a dynamic tool that can mimic the in vitro storage of RBC. Moreover, the system allows the control of gas supply which enables to probe the metabolism under aerobic and anaerobic conditions.
Such approach will provide information on the storage of in vitro storage and the impact on transfusion as well as on the fundamental mechanism involved in RBC aging.